Heat transfer mechanisms are simply ways by which thermal energy is transferred between objects. It is based on the basic principle that kinetic energy tries to be at equilibrium or equal energy states. There are three different ways for heat transfer to occur namely conduction, convection, and radiant heat. There is one more related phenomenon that transfers latent heat called evapotranspiration.
Conduction
Conduction heat transfer
is energy transport due to molecular motion and interaction. Conduction heat
transfer through solids is due to molecular vibration. Fourier determined that
Q/A, the heat transfer per unit area (W/m2) is proportional to the
temperature gradient dT/dx. The constant of proportionality is called the
material thermal conductivity k.
`\frac QA=-k\frac{dT}{dx} ...(1)`
Table.1: Thermal
Conductivities of Common Materials
In conduction, the
molecules simply give their energy to adjacent molecules until equilibrium is
reached. Conduction models do not deal with the movement of particles within
the material. The thermal conductivity k depends on the material, for example,
the various materials used in engines have the thermal conductivities (W/m K)
as given in Table.1. The thermal conductivity also depends somewhat on the
temperature of the material.
 |
| Fig.1.Conduction through Piston Cylinder Wall |
For a cast iron 0.012 m cylinder block at steady state, Fig.1, and at T1 = 100 °C and T2 = 300 °C heat transfer is given by equation (2)
`\frac QA=-k\frac{dT}{dx}=\frac{-k(T_1-T_2)}{\triangle x} ...(2)`
`=\frac{-80(100-300)}{0.012}`
= 1.3 MW/m2
Convection
Convection heat transfer
is energy transport due to bulk fluid motion. This type of heat transfer
through gases and liquids from a solid boundary results from the fluid motion
along the surface. Newton determined that the heat transfer/area (Q/A), is
proportional to the fluid-solid temperature difference (Ts − Tf).
The temperature difference usually occurs across a thin layer of fluid adjacent
to the solid surface. This thin fluid layer is called a boundary layer. The
constant of proportionality is called the heat transfer coefficient (h).
`\frac QA\propto T_s-T_f ...(3)`
`\frac QA=h(T_s-T_f) ...(4)`
The movement of the
thermal energy in convection is due to the movement of hot fluid. Usually, this
motion occurs as a result of differences in density. Warmer particles are less
dense, so particles with higher temperatures will move to regions where the
temperature is cooler and the particles with a lower temperature will move to
areas of higher temperature. Thus, the fluid will remain in motion until
equilibrium is reached. The heat transfer coefficient depends on the type of
fluid and the fluid velocity. The heat flux depending on the area of interest
is local or area-averaged. The various types of convective heat transfer are
usually categorized into the following areas:
Table.2:
Convective Heat Transfer Coefficients
|
Convection
type |
Description |
Heat Transfer Coefficients (h) (W/m2K) |
|
Natural
convection |
Fluid
motion is induced by density differences |
10
(gas) and 100 (liquid) |
|
Forced
convection |
Fluid
motion is induced by pressure differences from a fan or pump |
100
(gas) and 1000 (liquid) |
|
Boiling |
Fluid
motion is induced by a change of phase from liquid to vapor |
20,000 |
|
Condensation |
Fluid
motion is induced by a change of phase from vapor to liquid |
20,000 |
For a cylinder block,
Fig.2, with forced convection (h) of 1000, the surface temperature of 100 °C,
and a coolant temperature of 80 °C, the local heat transfer rate is calculated
as:
 |
| Fig.2: Convection Heat Transfer |
`\frac QA=1000(100-80)`
= 20000 W/m2
Radiation
Radiation heat transfer
is energy transport due to the emission of electromagnetic waves or photons
from a surface or volume, Fig.3. All moving charged particles emit
electromagnetic radiation. This emitted wave will travel until it hits another
particle. The particle that receives this radiation will receive it as kinetic
energy. Particles will receive and emit radiation even after everything is at
the same temperature, but it is not noticed because the material is at
equilibrium at this point. The radiation does not require a heat transfer
medium and can occur in a vacuum.
The heat transfer by
radiation is proportional to the fourth power of the absolute material
temperature. The proportionality constant is the Stefan-Boltzmann constant ≈
5.67 × 10−8 W/m2 K4. The radiation heat
transfer also depends on the material property emissivity (e) of the material.
`\frac QA=\varepsilon\times\sigma\times T^4 ...(5)`
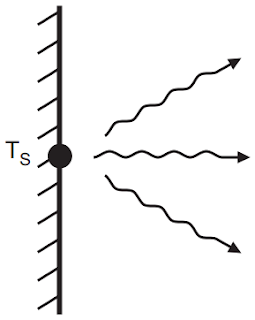 |
| Fig.3: Radiation Through Piston Cylinder Wall |
For a surface with an
emissivity of e = 0.8 and T = 373 K (100 °C), the radiation heat transfer is
`\frac QA=0.8\times5.67\times10^{-8}\times(373)^4`
= 878 W/m2
For moderate (less than
100 °C) temperature differences, it should be noted that the radiation and
natural convection heat transfer are about the same.
Evapotranspiration
Evapotranspiration is the
energy carried by phase changes, like evaporation or sublimation. Water takes a
fair amount of energy to change phase, so this process recognizes that water
vapor has a fair amount of energy associated with it. This type of energy
transfer mechanism is often not listed among the different types of transfer
mechanisms as it's harder to understand.
Make sure you also check our other amazing Article on : Fourier’s Law