Historically distillation was used since 1200 BC in perfumery operations. Early forms of distillation were batch processes using one vaporization and one condensation. Purity was improved by further distillation of the condensate. Greater volumes were processed by simply repeating the distillation. Chemists were reported to carry out as many as 500 to 600 distillations to obtain a pure compound. In the early 19th century the basics of modern techniques including pre-heating and reflux were developed. In 1877 U.S. Patent was granted for a tray column for distillation of ammonia and in the subsequent years for oil and spirits. With the emergence of chemical engineering as a discipline at the end of the 19th century, scientific rather than empirical methods were applied. More accurate designs were used during developments in petroleum industries. Today availability of powerful computers has allowed direct computer simulation of distillation columns.
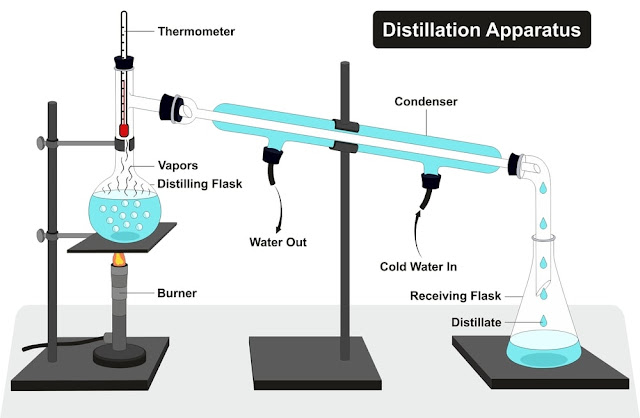
Fig.1: Basic Distillation Unit Set-Up
Distillation is one of
the most important processes for separating the components of a solution. The
solution is heated to form a vapour of the more volatile components in the
system, and the vapour is then cooled, condensed, and collected as drops of liquid,
Fig.1. By repeating vaporization and condensation, individual components in the
solution can be recovered in a pure state. Essences and many pure products from
the oil refinery industry are processed via distillation.
The objective of Distillation
The basic objective of
distillation is to separate the liquid mixture into two or more components. In
a basic distillation column, a feed stream enters in the middle of the column
and two streams leave, one at the top and one at the bottom. Components with lower
boiling points are concentrated in the stream leaving the top while components
with higher boiling points are concentrated in the stream leaving the bottom.
Separation is achieved by controlling the column temperature and pressure to
take advantage of differences in the relative volatility of the mixture
components and therefore tendency to change phase. The lighter, lower boiling
point components evaporate and travel up the column to form the top product and
the heavier, higher boiling point components condense and travel down the
column to form the bottom product.
Basic Principles of Distillation
Distillation is a process
by which a liquid mixture is separated into fractions with higher
concentrations of certain components by exploiting differences in relative
volatility. Distillation has been used widely to separate volatile components
from non-volatile compounds. In industrial settings such as oil refineries and
natural gas processing plants, this separation process is undertaken using a
distillation column.
The mechanism involved in
distillation is the differences in volatility between individual components.
With sufficient heat applied, vapours are formed from the liquid solution. The
liquid product is subsequently condensed from the vapour phase by removal of
the heat. Therefore, heat is used as the separating agent during distillation.
In general, distillation can be carried out either with or without reflux
involved. For the case of single-stage differential distillation, the liquid
mixture is heated to form a vapour that is in equilibrium with the residual
liquid. The vapour is then condensed and removed from the system without any
liquid allowed to return to the still pot. This vapour is richer in the more
volatile component than the liquid removed as the bottom product at the end of
the process. However, when products of much higher purity are desired, part of
the condensate has to be brought into contact with the vapour on its way to the
condenser and recycled to the still pot. This procedure can be repeated many
times to increase the degree of separation in the original mixture. Such a
process is normally called "rectification."
Fractionation:
It is another term for distillation also called fractional distillation.
Feed:
The liquid and/or gas feed into the distillation column.
Feed tray:
The tray below the inlet nozzle is called the feed tray.
Heavy Component:
The component with the lower relative volatility, for example, simple
hydrocarbon, is a component with a higher molecular weight. It is found in
higher concentrations in the bottom product of the column.
Light Component:
The component with the higher relative volatility, for example, simple
hydrocarbon, is a component with a lower molecular weight. It is found in
higher concentrations at the top of the column.
Stripping section:
It is a section that consists of trays between the bottom of the column and the
feed tray. In the stripping section, the aim is to concentrate the heavier
component in the liquid phase.
Rectifying section:
It is a section that consists of trays between the feed tray and the top of the
column. In the rectifying section, the aim is to concentrate the lighter
component in the vapour phase.
Top Product:
It is a product that leaves from the top of the column, also called the distillate.
This product is usually passed through a heat exchanger and liquefied.
Bottom Product:
It is the product that leaves through the bottom of the distillation column.
Reflux:
A portion of vapour from the top of the column has been condensed to a liquid
and returned to the column as a liquid above the top tray.
Reboiler:
A heat exchanger at the bottom of the column which boils some of the liquid
leaving the column. The vapour generated returns to the column at the bottom of
the stripping section.
Vapour-Liquid Equilibrium
(VLE) Curve: A plot of the actual composition of the
lighter component in the vapour phase for a given composition in the liquid
phase. Usually, it is derived from thermodynamic data.
Zeotropic mixture:
It is a mixture of liquids with different boiling points. For example,
nitrogen, methane, ethane, propane etc.
Azeotropic mixture:
It is a mixture of two or more liquids that has a constant boiling point because
vapour has the same composition as a liquid mixture.
Principle of Separation
Distillation takes
advantage of the difference in relative volatility of the feed mixture
components. Generally for two or more compounds at a given pressure and
temperature, there will be a difference in the vapour and liquid compositions
at equilibrium due to component partial pressure. Distillation exploits this by
bringing liquid and gas phases into contact at temperatures and pressures that
promote the desired separation. During this contact, the components with the
lower volatility (typically lower boiling point) preferentially move into the
liquid phase while more volatile components move into the vapour phase.
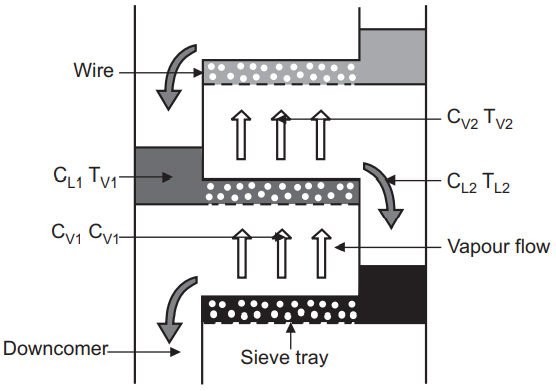
A distillation column may
use either trays or a packed bed to bring the gas and liquid into contact. For
a column using trays, we can consider the changes to gas and liquid phase
compositions as they both enter and exit a single tray. The liquid entering the
tray will contact the gas exiting the tray, Fig.2. The hotter vapour phase
heats the incoming liquid phase as it bubbles through the tray, evaporating the
light components which then leaves the tray with the vapour phase. Conversely,
the cooling of the vapour phase by the liquid phase will cause the heavier components
of the vapour phase to condense and exit the tray with the liquid phase.
Fig.2: Principle of Separation
For the liquid across the
tray:
CL1, heavy <
CL2, heavy
CL1, light >
CL2, light
TL1 > TL2
For the vapour through
the tray:
CV1, heavy >
CV2, heavy
CV1, light <
CV2, light
TV1 > TV2
Where, C is concentration
and T is temperature.
When packing is used
rather than trays the principle remains the same packing is often referenced in
terms of the height equivalent to a theoretical plate (HETP) i.e. what height
of packing is equivalent to one theoretical plate. The packing is just an
alternative method to bring the liquid and vapour phases into contact with the
liquid generally flowing over the surfaces of the packing material, while the
vapour passes up through the space between packing elements.
Typical Operating Parameters
The distillation process
can be improved by understanding the following operating parameters.
(i) Temperature:
The basic temperature profile of a distillation column is hotter at the bottom
and cooler at the top. For a simple two-component distillation the temperature
at the bottom is just lower than the boiling point of the heavier component.
The temperature at the top of the column is just above the boiling point of the
lighter component. To have a heavy component remain as a liquid at the bottom
of the column and the lighter component to stay as a gas we set the temperature
at the bottom to match this requirement. This temperature is set by adding heat
via a heat exchanger. Typically the heat added to the bottom of the column is
easy to control, via steam or hot oil flow rates.
At the top of the column,
the situation is reversed. The light component remains as gas while the heavier
component is condensed to a liquid and falls back down the column. The top
temperature is set just above the boiling point of the lighter component. The
temperature control situation is different at the bottom of the column because
the top product is to be a liquid when we send it for storage. All of the gas
coming out of the top of the column is condensed to a liquid. This liquid
stream is split with some returning to the column and some going to storage.
The top temperature is often controlled by changing the reflux rate, i.e. the
flow rate of liquid sent back to the top of the column. A higher reflux rate
means cooler liquid falling down the column against the rising warmer gas, and
the top temperature is lower. Overall heat is added at the bottom of the column
and heat is extracted at the top of the column. Inside the column, the
temperature balance is created between the hot gas rising the column and the
cooler liquid falling down the column.
(ii) Pressure:
There is typically a pressure gradient across the column with the pressure
being higher at the bottom of the column than the top. This pressure gradient
occurs as the liquid coming down the column hampers the flow of vapour up the
column and imposes a pressure loss on the flow. In steady-state distillations,
the pressure in the column is held constant, and the temperature is varied to
control the composition of the product streams.
Applications of Distillation
- Distillation is used for many commercial processes, such as the production of gasoline, distilled water, xylene, alcohol, paraffin, kerosene, and many other liquids.
- Distillation is used for purifying solvents and liquid reaction products.
- It is used in the manufacturing of distilled water, double distilled water used in Water for Injection and other pharmaceutical preparations.
- Toxic and costly organic solvents are used in the extraction, synthesis and analysis of drugs. These solvents can be recovered by distillation for economic as well as environmental protection benefits.
- Distillation is used in the separation of volatile oils such as clove oil, anise oil, cardamom oil, eucalyptus oil etc. from the plant extracts.
- It can be used in the separation of volatile components from a mixture of two or more volatile liquids.
- It can be used as a quality control method for alcohol content in liquid formulations. The alcohol is separated from formulations by distillation and alcohol content is determined.
- It can be used to liquefy and separate gases from the air. For example: nitrogen, oxygen, and argon are distilled from the air.
- Distillation is used in crude fermentation broths to separate alcoholic spirits.
- It can also be used in the fractionation of crude oil into gasoline and heating oil.
Make sure you also check our other amazing Article on : Multiple Effect Evaporator