Simple distillation is a unit operation in which two liquids with different boiling points are separated.
Principle of Simple Distillation
Simple distillation is a
process of heating and cooling liquids to separate and purify them. As the
liquid being distilled is heated, the vapours that form are richest in the
component of the mixture that boils at the lowest temperature. Purified component
boils, and thus turns into vapours, over a relatively small temperature range
(2 or 3 °C). A careful observation of the temperature in the distillation flask
helps to carry out a good separation. As distillation progresses, the
concentration of the lowest boiling component steadily decreases. Eventually,
the temperatures within the apparatus begin to change and a pure compound is no
longer being distilled. As the temperature continues to increase the boiling
point of the next-lowest-boiling compound is approached. When the temperature
again stabilizes, another pure fraction of the distillate can be collected.
This fraction of distillate is primarily the compound that boils at the second-lowest
temperature. This process can be repeated until all the fractions of the
original mixture are separated. For simple distillation to perform, the two
liquids’ boiling points must have a difference of at least 25 °C (or about 77
°F).
Construction of Simple Distillation
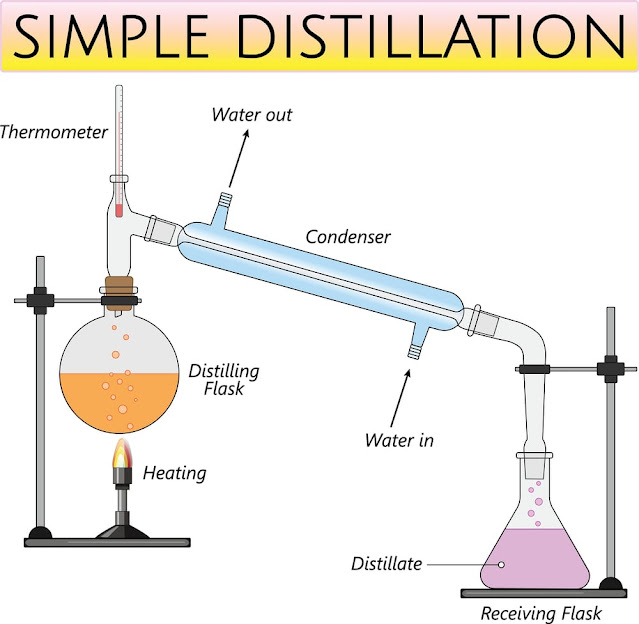
The set of simple
distillation consists of a distillation flask with a sidearm sloping downwards,
Fig.1. The mouth of the flask is fitted with a cork closure with an inserted
thermometer. The condenser is attached to the sloping arm for cold water
circulation with an inlet on the lower side and an outlet at the upper side.
The cold water pipe is attached to the inlet while the outlet discharges water
to waste. The condenser outlet delivers a liquid product that is collected in a
collector or receiver.
Working of Simple Distillation
1. Calibration of
thermometer: Calibration can be done by placing the
thermometer in an ice bath of distilled water. Allow the thermometer to reach
thermal equilibrium. Now remove from ice water and place it in a beaker of
boiling distilled water and again allow it to reach thermal equilibrium. If the
temperatures measured does deviate from the expected values by more than two
degrees then use it for recording temperature in the distillation process.
2. Filling the
distillation flask: The flask is filled with not more than
two-thirds of its volumes to have sufficient space above the liquid surface so
that when boiling begins the liquid will not be propelled into the condenser.
This is important in the viewpoint of the purity of the distillate. Porcelain
chips should be placed in the distillation flask to prevent superheating of the
liquid and to cause a more controlled boiling, eliminating the possibility of
liquid bumping into the condenser.
3. Heating the
distillation flask: The distillation flask is heated slowly
until the liquid begins to boil. The vapours rise through the neck of the
distillation flask and pass through the condenser and condense and drip into
the collection receiver, Fig.1. Generally, the rate of distillation is
approximately 20 drops per minute. Distillation must occur slowly enough that
all the vapours condense to liquid in the condenser. Many organic compounds are
flammable and if vapours pass through the condenser without condensing, they
may ignite as they come in contact with the heat source.
4. Condensation of
vapours: As the distillate begins to drop from the condenser,
the temperature changes steadily. When it is stable, a new receiver is used to
collect all the drops that form over a two to a three-degree range of
temperature. As the temperature begins to rise further, a third receiver is
used to collect the distillate. This process is repeated; using a new receiver
every time the temperature stabilizes or begins changing until all of the
distillate has been collected in discrete fractions. All fractions of the
distillate should be saved until it is shown that the desired compound has been
effectively separated by distillation.
 |
| Fig.1: Simple Distillation |
Handling Precautions
- If direct heating is used stop the heat source from the distillation flask before all of the liquid is vaporized.
- When all of the liquid is evaporated, the temperature of the glass of the distillation flask rises very rapidly, possibly igniting whatever vapours still are present in the distillation flask.
- Never distil to dryness. The residue left in the distillation flask may contain peroxides, which could ignite or explode after all the liquid has distilled away.
- Make sure that all joints are secured very tightly. If any vapour escapes at the connection points, it may come into direct contact with the heat source and ignite.
- Never heat a closed system, the increasing pressure will cause the glass to explode.
- If the distillation flask has a tapered neck, the thermometer may be placed in such a way as to block the flow of vapours up the neck of the flask; in effect creating a closed system; make sure that if using a tapered neck flask, the thermometer is not resting in the lowest portion of the neck.
- If the liquids comprising the mixture that is being distilled have boiling points closer than 25 °C to one another, the distillate collected will be richer in the more volatile compound but not to the degree necessary for complete separation of the individual compounds.
Advantages of Simple Distillation
- It is a simple, cheap, easy and economic method.
- It requires less energy.
- This process requires a single run and thus is comparatively faster.
Disadvantages of Simple Distillation
- The final product may contain impurities.
- Azeotropic mixtures cannot be separated by simple distillation.
- Not suitable for mixtures containing thermolabile components.
- The volume of the mixture should be no more than 2/3rd of the container.
Applications of Simple Distillation
- Simple distillation is primarily used for the production of distilled water.
- Many volatile oils are separated by simple distillation.
- It is also used in the separation of organic solvents from mixtures.
- It is used to separate non-volatile components from volatile ones.
- It is used in preparing pharmaceutical spirits.
Make sure you also check our other amazing Article on : Distillation